Water - Pharmaceuticals in the environment
Building a healthier and more environmentally sustainable future
The European pharmaceutical industry is committed to ensuring clean water for Europeans. Companies have invested heavily in ensuring that effluent from manufacturing process do not enter the water system. However, when patient take essential medicines from cancer treatments to paracetamol, these pass through the patients system and make their way into waste water. We continue playing an active role in mnimising the impact of medicines on the environment while safeguarding access to effective treatments for patients is a critical issue across all sectors of healthcare.

At EFPIA, we believe that a collaborative approach allows us to increase our mutual knowledge and understanding on how to proactively address any potential risks imposed by the presence of PiE. To this end, EFPIA, AESGP and Medicines for Europe have developed the Eco-Pharmaco-Stewardship (EPS) framework that applies the widely accepted principles of product stewardship and is implemented across the industry and with broader stakeholders in the healthcare and environmental sector.
Multiple initiatives under the Eco-Pharmaco-Stewardship have contributed over the last couple of years to improving scientific understanding, finding new ways to detect the trace amounts of PiE, understanding their impact, prioritising APIs posing a potential risk to the environment and also further reducing discharges from manufacturing plants. As an industry, we are striving to continually enhance our processes to deliver life-saving treatments in ways that also protect of the environment.
- Review of Commission approach to allocating toxic load to pharmaceuticals
- Eco-Pharmaco-Stewardship (EPS) initiative – Care for people & our environment
- Balancing challenges onEnvironment with access to medicines in Europe
- Interassociation paper on extended environmental risk assessment
- Joint statement on the European Parliament’s Resolution and on the Strategic Approach to PiE
- Joint Statement on the EU Strategic Approach to PiE
- Industry contribution to a Strategic Approach to PiE – guest blog
- Joint declaration on Pharmaceuticals in the Environment
- Q&A on pharmaceuticals in the environment
Our commitment to addressing water pollution
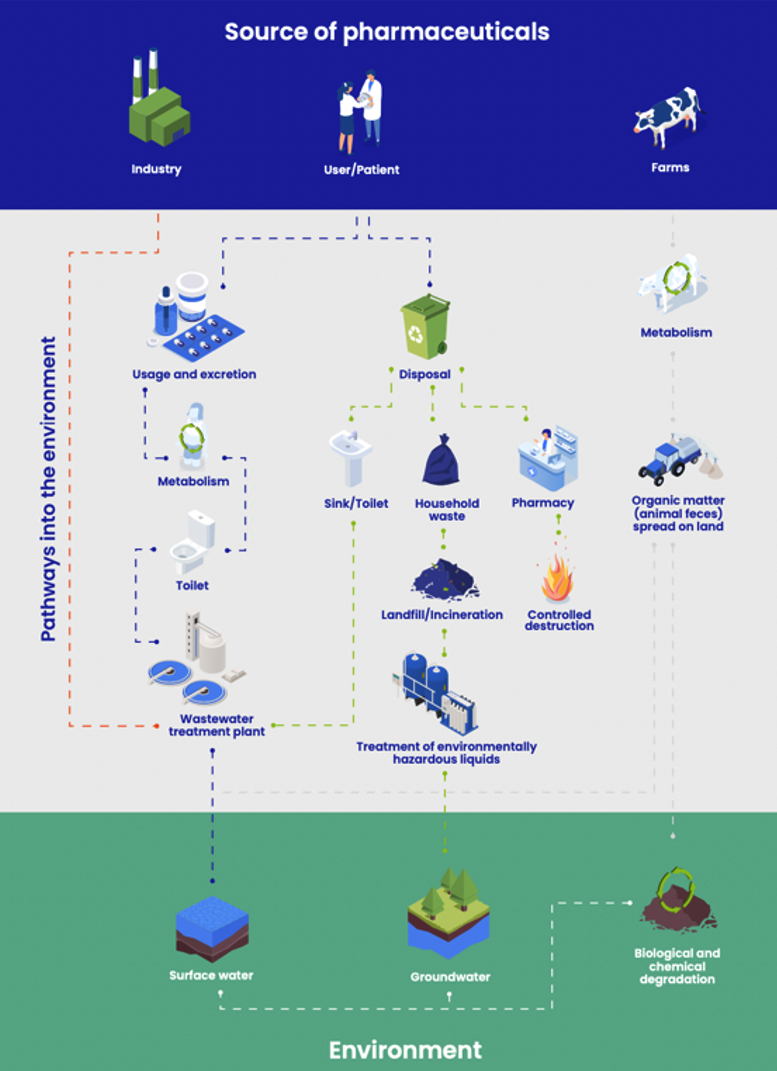
As an industry, we take our responsibility for water pollution seriously. We already treat water from our manufacturing processes, and over the past 15 years, we have worked to reduce micro-pollutants from medicines, disposal, and manufacturing. However, some pollutants inevitably enter European wastewater through the everyday use of medicines—whether by excretion or, to a lesser extent, from the incorrect disposal of unused or expired medicines. We recognize the environmental impact of this and are committed to paying our fair share to address it.
While we fully support the ambition of the Urban Wastewater Treatment Directive to reduce the impact of pharmaceuticals in wastewater, the decision to single out only the pharmaceutical and cosmetic industries to fund the clean-up of all micropollutants across Europe is arbitrary, ineffective, and unfair. EFPIA firmly supports the Polluter Pays Principle, ensuring that all industries responsible for pollution bear their fair share of the costs. As an industry we are happy to pay our fair share. The Directive, as currently designed, undermines the ambitions of the Green Deal by completely failing to incentivize other sectors to reduce micro-pollutants in the water.
We remain committed to working towards the implementation of the Directive in a fair, predictable, proportionate, and non-discriminatory way to achieve meaningful environmental progress.

Extended Environmental Risk Assessment
Every new pharmaceutical requires an Environmental Risk Assessment (ERA) before it can be approved in the EU. EFPIA supports science-based risk assessments for medicines entering the market.
The extended E.R.A. (eERA) approach is designed to address challenges and strengthen the E.R.A. process in the EU by providing the following benefits:
- An API based E.R.A. which better reflects the risks posed to environment from patient use
- Strengthen the industry’s commitment to conduct robust and risk-based E.R.A.s without compromising environmental protection or patient access to medicines
- Provision for the ability to automatically cross-reference E.R.A. data in marketing authorisation applications
- Provide a mechanism for risk identification, refinement, and management during the MAA evaluation process
- Provide clarity on appropriate well-defined follow-up responsibilities for E.R.A.s with no need for independent and duplicative risk identification and prioritisation processes under different legislations (e.g. Water Framework Directive)
- Updates to the E.R.A. across the life cycle of the API in each MP in which it is contained that will ensure that each E.R.A. reflects the latest environmental information
- A focus on risk that reduces the burden on regulators (i.e. oversight) and industry
- Reduction in the duplication of testing, delivering improved E.R.A. consistency, proportionate use of testing resource, and bioethical benefits
- Suggestions for mechanisms to increase the transparency of, and access to, E.R.A. data

Responsible Manufacturing Effluent Management
Ensuring the use of appropriate environmental risk management measures to adequately control manufacturing effluent emissions is an important area of focus for the pharmaceutical industry and is an approach already in place in a number of companies as described by Caldwell et al. (2016). The pharmaceutical industry demonstrates its engagement for sound manufacturing effluent management by developing and implementing initiatives, which address the potential risks of discharges of APIs from manufacturing operations. Compliance with local laws, regulations and environmental permits is a prerequisite for all API and drug product manufacturing operations. Additionally, the member companies of AESGP, EFPIA and Medicines for Europe – as part of the PIE inter association taskforce - have developed a set of principles for responsible effluent management for their own, and supplier, manufacturing sites which focus on the following areas:
- Compliance with applicable company standards
- Implementation of defined wastewater management programs that are based on risk management and good engineering principles
- Definition of site and API specific discharge targets based on safe concentrations in the receiving surface waters
- Discharge of manufacturing wastewater containing API must have an environmental risk assessment
- Interassociation Policy statement on responsible manufacturing effluent management
- Technical guidance document on responsible manufacturing effluent management
- Slide set on the guidance, including real world examples
Continued investment in filling the knowledge gap through further research and innovation
The pharmaceutical industry, in cooperation with academia, regulators, public entities and the European Commission, has been working to close the knowledge gaps on the environmental risk of medicines, through the IMI/IHI project . By facilitating access to data and tools to regulators, environmental organisations and policy makers, a new standard for environmental protection across Europe can be adopted. The evidence-based information system and tools generated by PREMIER will give regulators, water managers, and the scientific community access to valuable knowledge that can be used to advance on the responsible use of medicines and mitigate impacts on the environment.
AMR Industry Alliance
The Pharmaceutical Industry is supportive of a circular approach to its operations and products and is aligned with the European Commission's Circular Economy Action Plan. We will achieve the goals of the Circular Economy Action Plan concurrent with our aspiration to safeguard the future supply of pharmaceuticals for patients and improve human health.
The pharmaceutical industry’s approach to circularity builds on our long experience in environmental sustainability, while recognizing the constraints (e.g. speed of transition), from operating in a highly regulated industry. Circularity and regulation of pharmaceuticals should be carefully balanced. The innovation to enable circularity will drive new opportunities for growth, promote greater resource efficiency, create a more competitive economy and reduce pollutants.
Implementation of a circular economy is fundamental to help limit the global temperature increase to less than or equal to 1.5C, and we welcome the opportunity to be part of the solution by working collaboratively with the EU in shaping the legislative framework and within our organizations to mitigate our impacts.
 #MEDSDISPOSAL – EASIER THAN YOU THINK
#MEDSDISPOSAL – EASIER THAN YOU THINK
The pharmaceutical industry stands ready to support the EU and Member States’ communication activities and awareness raising campaigns on the appropriate use, storage and disposal of medicines. Most European countries have special medicines disposal schemes in place in order to prevent medicines from ending up in the environment. To visualise these, several healthcare stakeholders (industry, healthcare professionals and student organisations) jointly developed #medsdisposal.eu. This is an online communication campaign aimed at raising public awareness on the existing collection and disposal schemes emphasizing the fact that it is everyone’s responsibility and it is easier than one may think.21
The disposal schemes for unused medicines have a great potential to eliminate some traces of PiE, (originating from incorrect disposal of expired or unused medicines), by implementing a cost-effective, efficient and straightforward take-back process. The pharmaceutical industry has been discussing with other partners along the value chain opportunities to further explore the potential of collection schemes for unused medicines since they are an important part of the solution to the ongoing discussion around the extended producer responsibility.

