Assessing the added value of medicines to support access: the benefits of European cooperation
13.01.16
“Meaningful” information on a medicine covers many aspects, from economics to ethics. So HTA uses a broad range of analyses to cover these aspects, including relative efficacy assessment and relative effectiveness assessment. Both look at the medical or therapeutic added value of medicines, compared with alternatives, either in a controlled, trial-based environment (relative efficacy assessment) or in real-life (relative effectiveness assessment).
So What is HTA in a Nutshell?HTA is a multidisciplinary process that summarises information about the medical, social, economic and ethical issues related to the use of a health technology, in a systematic, transparent, unbiased and robust manner.
What about Relative Efficacy / Relative Effectiveness?Relative efficacy may be defined as the extent – under ideal circumstances – to which an intervention does more good than harm, compared with one or more alternative interventions.
By contrast, relative effectiveness is essentially the extent – under the usual circumstances of health care practice – to which an intervention does more good than harm compared with one or more intervention alternatives.
So How Do HTA Agencies Work?Decision makers tend to request HTA agencies to collect and assess information across a wide range of areas. While it varies from country to country, this information focuses typically on the medical (relative efficacy and/or relative effectiveness assessment) and economic impact (for example cost-effectiveness assessment or budget impact analysis) of new pharmaceuticals on the individual patient and on the healthcare system as a whole.
Do All Assessments Achieve the Same Results?Surprisingly, HTA agencies reach different conclusions on the medical impact (relative efficacy and/or relative effectiveness assessment) of new pharmaceuticals, even though the data studied is predominantly the same for all markets – such as safety and efficacy data from registration trials. This is because HTA agencies adopt different approaches to rating and interpreting this data. This might apply to trial design, relevant endpoints, appropriateness of defined patient subgroups and treatment comparators.
Equally interesting is the fact that the views of HTA agencies may sometimes be out of step with the outcomes of the European Medicines Agency’s (EMA’s) review of a medicine.
It is clear that these discrepancies benefit neither patients, nor health systems. They are confusing, lead to avoidable redundancies and thereby to unnecessarily lengthier procedures. The negative impact on patient access to medicines is unquestionable.
The situation is different when it comes to economics. Economic evaluations rely on locally available data, so different results will be achieved in different countries. This is mainly because European countries enjoy varying economic circumstances and costs of alternative treatments, medical services associated with a condition, including the cost of medical care by healthcare professionals, are likely to diverge.
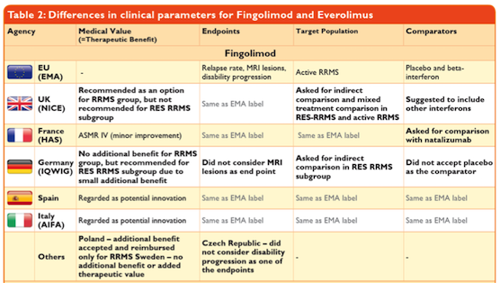
Source: Weber, S., Jain, M., Nallagangula, T. K., Jawla, S., Rai, N., Dev, D., & Cook, N. (2015, November). Heterogeneity in Relative Efficacy Assessments (REA) across European HTA Bodies: Opportunity for Improving Efficiency and Speed of Access to Patients? Poster presented at ISPOR 18th Annual European Congress, 7-11 November 2015, MiCo - Milano Congressi, Milan, Italy
What Mechanism Do We Have to Address these Hurdles?The EUnetHTA concept was set up in 2006, to promote more collaboration and harmonisation in the EU, by linking national HTA agencies, research institutions and health ministries. This allows for an affective exchange of information and lends support to policy decisions by European Member States. Over time, EUnetHTA has evolved via the mechanism of Joint Action between Member States and the European Commission (Joint Action 1 2010-2012, Joint Action 2 2012-2015, expected Joint Action 3 2016-2019).
EFPIA has been engaged with EUnetHTA since the start and supports its goal. EFPIA further supports the establishment of a permanent structure of cooperation following the conclusion of Joint Action 3 on HTA in 2020.
How is EUnetHTA Contributing in Practice?EUnetHTA is addressing divergences by conducting European assessments of the incremental therapeutic benefit of innovative medicines at the time of launch.
Already, a number of national HTA agencies are working together, together with a range of pharmaceutical manufacturers – all of whom are members of EFPIA.
Pilot projects have shown that through EUnetHTA interested stakeholders can collaborate successfully to produce European reports of relative efficacy at time of launch. However more needs to be done to make sure that national decision-makers accept and recognise these European reports and fully integrate their findings in national access pathways.
Is this Approach Really Effective?In 2015, EFPIA commissioned an independent review of the five pilots to review the progress of Joint Action 3. Conducted by Charles River Associates, the review makes clear recommendations on how stronger collaboration in the EU on relative efficacy assessments (REAs) can be achieved during Joint Action 3. These measures will benefit HTA agencies and ultimately patients across the whole of Europe. We believe that efficient and high quality European REAs of new pharmaceuticals at time of launch are essential. Let’s get on with it! This is the call addressed to Member States by representatives of patients, healthcare professionals, politicians, regulators, HTA agencies, and industry during the World Health Summit in 2015.
Decision-making on pricing, reimbursement and access should remain a national competence. If this were integrated effectively with European REAs, though, the result would issue a strong, positive signal to pharmaceutical companies concerning Europe’s healthcare system priorities.
Ultimately, it will have the potential to reduce time to patient access and strengthen the equity of care throughout Europe.